PERIODIC CLASSIFICATION OF ELEMENTS NOTES
Basic concepts –
Matter – It is anything around us which has mass and which occupy space
States of matter: –
- Solid
- Liquid
- Gas
- Plasma state (Occurs at extremely high temperature – 11,000° – 14,500° Fahrenheit or 150 million degrees Celsius)
- Bose Einstein condensates (temperatures very close to absolute zero i.e., 0 K or −273.15 °C or −459.67 °F).
Types of matter –
1)Pure substances
2) Mixture
Pure substances – Made of molecules having same types of atoms. It may be element or compound based on nature of its own constituents.
Elements – These are the molecules which are made up of only one type of atom.
They may be metals, nonmetals metalloids
Types:
i] Metals
ii] Nonmetals
iii] Metalloids
Characteristics of elements
- We cannot break them into simpler substances
- Simplest form of pure substance
- Cannot be separated into simpler substances by any physical or chemical method
- They may be metal nonmetal metalloids
Compounds – Substances produced by a chemical reaction of two or more elements.
Characteristics of compound
- Always made up of same elements combined in fixed proportion
- Formed due to chemical change
- Its properties are totally different from original substance
- Constituent elements can be only separated by chemical method
Mixture – Materials which consists of two or more pure substances (elements or compounds) which are not chemically combined
Characteristics of mixture
- Constituents are present in any proportion
- Original properties of substances do not change
- Constituents can be separated by physical methods
- Composition is variable
The element which was first discovered – Phosphorus discovered by Henning brand in 1649
Sir Lavoisier were the first one to classify elements into two parts – Metals and Non-Metals
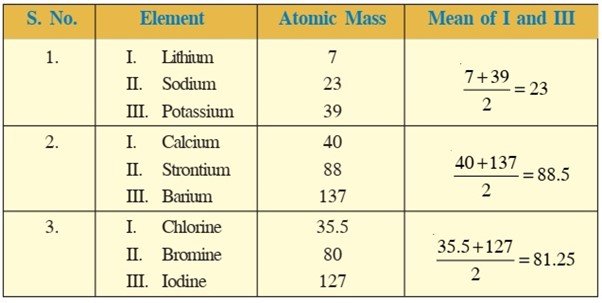
DOBEREINERS TRIADS –
- 33 elements
- In 1817
- German scientist
- Johann Wolfgang Dobereiner
Basic criteria to classify
- Arranged in increasing order of atomic mass
- Arranged in group of three
- The properties of these elements were having similar chemical properties and regular gradation and physical properties
Law – When elements with similar properties are arranged in increasing order of their atomic masses in a group of 3, the atomic mass of middle element was approximately equal to the mean of atomic mass of the other two elements
Limitations / Drawbacks of Dobereiner’s law –
- All known elements could not be classified in Dobereiner’s triad
- He could identify only four triads which are only 12 elements.
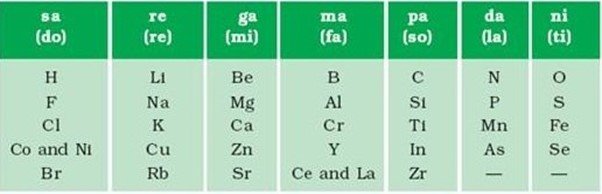
NEWLANDS LAW OF OCTAVES
- John Alexander Newlands
- In 1866
- English scientist
Basic criteria to classify
- Arranged elements in increasing order of their atomic masses.
- Compared this similarity with the octaves of music
- Elements having different chemical properties
LAW- When elements with different properties are arranged in horizontal rows in increasing order of the atomic masses then the properties of every 8th element were similar to that of the properties of the first element
For example – Sodium is the 8th element from Lithium and both have similar properties
Limitations / Drawbacks of Newlands law of octaves
- Applicable only up to calcium-
After calcium every 8th element didn’t show the properties similar to that of the 1st element
- Placed two elements in same box-
In order to fit existing elements according to their properties, he placed two elements at same place
Ex. Cobalt And Nickel, Cerium and Lanthanum
- Placed elements with different properties under same note-
Generally, elements in same note are having similar properties but at some places he placed elements with different properties in same note
Ex. i. Iron is a metal, and it is placed with oxygen and sulphur which are nonmetals.
Cobalt and Nickel are metals and are placed with halogens
4. Did not have provision to accommodate newly discovered elements –
The properties of elements which were discovered later on did not fit Newlands law of octaves
MENDELEEVS PERIODIC TABLE
- Russian chemist – Dmitri Evanovich Mendeleev – Professor of chemistry at University of Petersburg, Moscow.
- 63 elements were known
- Tried to correlate atomic masses of elements with their physical and chemical properties
- Among chemical properties, he mainly focused on the compounds formed by elements with hydrogen and oxygen (Hydrides and Oxides) because they are highly reactive and form compounds with almost all elements
- He took 63 cards and, on each card, he wrote properties of one element and sorted out the elements with similar properties, then he found that there is correlation of properties and their atomic masses.
Characteristics / features of Mendeleev’s Periodic Table –
- Periods –
It consists of 7 horizontal rows called – Periods. They are numbered from 1 to 7.
2. Groups –
- It consists of 8 vertical columns called groups.
- They are numbered as I to VIII.
- First seven groups are subdivided into sub groups A and B.
- The elements in subgroup A are called Normal elements.
- The elements in subgroup B are called Transition elements.
- The 8th group contains 9Transition elements which lie in 4th, 5th and 6th
Merits of Mendeleev’s Periodic Table –
- It is the first successful classification of all known elements.
- Correction of doubtful atomic masses – He revised atomic masses of some elements.
Ex. Atomic mass of Beryllium was considered 14.9u which was modified by Mendeleev as 9.4 u.
- Prediction of new elements and their properties-
Mendeleev kept some vacant places in the periodic table for elements which were not discovered. Also, he predicted their properties.
Ex. i. Eka boron à Scandium
- Eka aluminium à Gallium
iii. Eka silicon à Germanium
- Position of Inert gases/ Noble gases / zero group elements / rare gases – when noble gases were discovered, they were included in the periodic table without disturbing the position of other elements as zero group.
Limitations / Demerits of Mendeleev’s Periodic Table –
A) No fix position for Hydrogen – The properties of Hydrogen resemble with alkali metals and halogens.
a) Similarities with halogens –
- Just like halogens (F2, Cl2, Br2, I2) hydrogen (H2) also exists in diatomic state.
- Like halogens, Hydrogen also forms similar compounds with metals and non-metals.
| Element | Compounds with metals
(Ex. Na) |
Compounds with Nonmetals
(Ex. Carbon) |
| Hydrogen | NaH | CH4 |
| Chlorine (halogen) | NaCl | CCl4 |
b) Similarities with alkali metals – Like alkali metals, Hydrogen combines with oxygen, sulphur, halogens to form compounds having similar formulae.
| Element | Compounds of Hydrogen | Compounds of Alkali metal (Na) |
| Oxygen | H2O | Na2O |
| Chlorine | HCl | NaCl |
| Sulphur | H2S | Na2S |
B) Doubtfulness about certain pairs of elements –
Elements are arranged on basis of atomic masses and similarities of properties.
At some places, to arrange elements according to their properties, he has kept some elements with higher atomic mass before element with lower atomic mass.
Ex. Co (58.93u) is placed before Ni (58.71u)
C) Uncertainty in prediction of new elements –
The atomic masses do not increase in regular manner. Therefore, it is not possible to predict how many new elements could be discovered between two elements
D) No fixed position for isotopes –
- Isotopes are the elements having similar properties (as they have same atomic number) and their atomic masses are different.
- They were discovered long after Mendeleev.
- Since, the periodic table is arranged on the basis of increasing order of atomic mass, they should be placed at two different places.
- But as they have same properties, they should be placed in same position.
- Thus, their position is anomalous.
E) Different groups for similar elements –
In some cases, elements with similar properties are placed in different group.
Ex. Cu and Hg having similar properties are placed different groups.
F) Doubtfulness about position of elements –
Whole number of atomic masses of Co (58.93u) and Ni (58.71u) is same i.e., 59. So, there was doubtfulness regarding their sequence.
MODERN PERIODIC TABLE –
In 1913 A.D., Henry Moseley demonstrated that atomic number is the fundamental property of an element than atomic mass.
Modern periodic law – Properties of elements are periodic function of their atomic number.
Characteristics of MPT –
In this periodic table, classification of elements is done in increasing order of atomic numbers.
GROUPS –
- The elements in same group are having same number of valance electrons (same outer electronic configuration) and thus they have same valency. Hence, their properties are same.
Ex. Elements in group 1 –
H = 1
Li = 2, 1
Na = 2, 8, 1, etc. here, all the elements are having 1 electron in outer shell, hence, all are having valency 1, they tend to donate electrons, and all are metals.
- Electronic configuration of outer shell is characteristic of a particular group.
- It consists of 18 vertical columns – groups.
- Elements in groups 1 and 2, and in groups 13 – 18 are called normal / representative elements.
(Outer shell incomplete). Total 50 normal elements are present.
- Group 1 elements – Alkali metals
- Group 2 elements – Alkaline earth metals
- Group 13 elements – Boron family
- Group 14 elements – Carbon family
- Group 15 elements – Nitrogen family
- Group 16 elements – oxygen family
- Group 17 elements – Halogens
- Group 18 elements – inert gases/ noble gases
- Group 3 -12 elements – Transition elements
- (Properties are intermediate between properties of normal elements). Their two shells are incomplete. 40 transition elements.
Metalloids – The elements are broadly divided into metals and non-metals by a zigzag line running diagonally.
- Metals are towards left of the line and non-metals towards right.
- Elements like Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), Polonium (Po), are called metalloids as their properties resemble with metals and non-metals both.
PERIODS –
- In modern periodic table, there are 7 horizontal rows called periods.
- Elements in the same period have same number of shells.
- Period number = number of shell which starts filling.
- Elements in 1st period are having 1 shell.
Elements in 2nd period are having 2 shells…and so on……
- 1st period has 2 elements – shortest period. (H and He)
- 2nd period has 8 elements – short period. (Starts with Li (atomic number 3) to Neon (atomic number 10)
- 3rd period has 8 elements – short period. Starts with Na (atomic number 11) to Argon (atomic number 18)
- 4thperiod has 18 elements – long period. Starts with K (atomic number 19) to Krypton (atomic number 36)
- 5th period has 18 elements – long period. Starts with Rubidium (atomic number 37) to Xenon (atomic number 54)
- 6th period has 32 elements – longest periods. Starts with Caesium (atomic number 55) to Redon (atomic number 86)
- 7th period has 32 elements – longest periods. Starts with Francium (atomic number 87) to Oganessonon (atomic number 118)
- Transition elements –
In the middle of periodic table, there are 4 series of 10 elements each.
They lie in the 4th, 5th, 6th and 7th periods and 3 – 12 groups. These are transition elements having intermediate properties between normal elements.
Inner transition elements –
- In order to avoid the periodic table becoming lengthy, two series of 14 elements each have been placed at the bottom of periodic table.
- Lanthanides – The first series having atomic numbers 58 – 71 lying in gr 3 and 6th period. Starts with Cerium and ends with Lutetium.
- These are also called rare earth elements.
- Actinides – The second series having atomic numbers 90 – 103 lying in gr 3 and 7th period. Starts with Thorium and ends with Lawrencium.
- The actinides are radioactive.
- Thorium, protactinium and Uranium are natural radioactive substances while others are man made radioactive.
- The lanthanides and actinides together are called inner transition elements as they form a series within the transition elements.
- There are 28 inner transition elements.
PERIODIC TRENDS –
Elements in groups and periods show certain regularity in their properties. This is called periodic trends.
- Valency – It is the combining capacity of an element.
Variation of valency in group – All elements in same group have same outer electron configuration (valance electrons) therefore, their valency is same.
Ex. Elements in group 1 – valency 1
Group 2 – valency 2
Group 13 – 3
Group 14 – 4
Group 15 – 3
Group 16 – 2
Group 17 – 1
Group 18 – 0
Ex. Group 1
| Element | Atomic number | Electronic configuration |
| H | 1 | 1 |
| Li | 3 | 2,1 |
| Na | 11 | 2,8,1 |
| K | 19 | 2,8,8,1 |
| Rb | 37 | 2,8,18,8,1 |
| Cs | 55 | 2,8,18,18,8,1 |
| Fr | 87 | 2,8,18,32,18,8,1 |
Variation of valency in periods – On moving from left to right in periods 2 and 3, the valency first increases from 1 to 4 and then decreases from 4 to 0
Ex. Elements in third period.
| Atomic number | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Symbol | Na | Mg | Al | Si | P | S | Cl | Ar |
| Electronic configuration | 2,8,1 | 2,8,2 | 2,8,3 | 2,8,4 | 2,8,5 | 2,8,6 | 2,8,7 | 2,8,8 |
| Valance electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Valency
|
1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Atomic size – It is determined by atomic radius.
Atomic radius is the distance between centre of atom and its outermost shell.
Its unit is picometer (pm)
1pm = 10 -12 m
Variation of atomic size in groups –
- On moving down a group, the atomic radius gradually increases.
- As new shells are added in each succeeding element, the number of shells increases.
- As a result, the distance between the nucleus and outermost shell increases and thus atomic size increases from top to bottom.
http://PERIODIC CLASSIFICATION OF ELEMENTS NOTES
| Element | Symbol | Shells | Atomic size |
| Lithium | Li | K, L |
|
| Sodium | Na | K, L, M |
|
| Potassium | K | K, L, M, N |
|
Variation of atomic size in periods –
- On moving from left to right in a period, the atomic radius decreases.
- As, atomic number increases by 1, it means the number of protons and electrons increases by 1.
- The addition of electrons takes place in same shell.
- This increases the nuclear charge by 1 in each succeeding element.
- Due to this, the electrons are attracted closer to the nucleus and hence, the atomic size decreases.
Ex. Atomic radii of elements in period 2
| Element | Li | Be | B | C | N | O | F | Ne |
| Atomic radius |
Position of the elements in the periodic table / How different limitations of Mendeleev’s periodic table are removed by MPT
The modern periodic table is arranged according to increasing order of atomic numbers.
The modern periodic table explains the main limitations of Mendeleev’s table as follows.
Anomalous position of some pairs of elements-
- In Mendeleev’s periodic table, cobalt (58.93 u) is placed before nickel (58.71 u) in order to place the elements according their properties.
- The modern periodic table is arranged according to increasing order of atomic numbers.
- As, the atomic number of Cobalt is 27 and that of Nickel is 28, therefore Cobalt is placed before Nickel in MPT.
Position of isotopes –
- Isotopes are having same properties and different atomic masses. So, there was confusion related to their position in Mendeleev’s periodic table.
- Modern periodic table is based on atomic numbers and not atomic mass.
- All the isotopes have same atomic number.
- Therefore, they can be placed at same place in same group.
- the isotopes of Chlorine, Cl – 35, Cl-37 are placed at same place. Similarly, isotopes of Hydrogen, H-1, H-2, H-3 are also placed in same group at same place.
Position for newly discovered elements –
- Since, rise in atomic mass was not uniform while going from one element to the next, it was not possible to predict how many elements could be placed in between the known elements.
- The atomic numbers increase by one from one element to the next in MPT. Therefore, the number of new elements to be discovered in between known elements is equal to difference in their atomic numbers.
- Here, atomic number of Chromium is 24 and that of manganese is 25. So, there could not be any element in between these two.
Position of Hydrogen –
- Hydrogen has same outer electronic configuration (has 1 electron in outer shell) like alkali metals and has tendency to donate electron and form positively charged cation. (H+)
- Also, it has lowest atomic mass and lowest atomic number.
- Due to these reasons, it is placed with alkali metals and not with halogens
Electronic Configuration of elements in MPT-
In Groups –
- All the elements in any group of the periodic table have identical outer shell configuration.
- As properties of any element depend upon its valance shell electronic configuration, therefore elements in a group have similar properties.
In periods –
| Atomic number | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Symbol | Na | Mg | Al | Si | P | S | Cl | Ar |
| Electronic configuration | 2,8,1 | 2,8,2 | 2,8,3 | 2,8,4 | 2,8,5 | 2,8,6 | 2,8,7 | 2,8,8 |
| Valance electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Valency
|
1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
- From the table, it is clear that as we move from left to right in the 2nd and 3rd periods, the number of valance electron increases by one unit 1 to 8 as the atomic number increases by one unit. However, the number of shells containing electrons remains the same.
- Each period starts with filling electrons in new shell.
Metallic and non-metallic characteristics –
- Generally, metals have 1,2 or 3 electrons in their valance shell so, they have tendency to donate electrons and form positively charged cations.
- Therefore, the metals are also called electropositive.
- Electro positivity – Tendency to donate electrons and form positively charged cations.
- Nonmetals generally have 4 to 8 electrons in their outermost shells so, they have tendency to accept electrons and form negatively charged anions.
- Therefore, the non-metals are also called electronegative.
- Electro negativity – Tendency to accept electrons and form negatively charged anions
Variation in groups –
On moving down a group, atomic size gradually increases. As a result, the force of attraction between the nucleus and valance electrons decreases. Hence, the metallic or electropositive character increases as we move down a group.
OR
The non-metallic or electronegative character decreases as we move down a group
Group 1
| Element | Symbol | Metallic character |
| Hydrogen | H | Least electropositive
(Least metallic character) |
| Lithium | Li | |
| Sodium | Na | |
| Potassium | K | |
| Rubidium | Rb | |
| Caesium | Cs | |
| Francium | Fr | Most electropositive
(Most metallic character) |
Group 17
| Element | Symbol | Non-metallic character |
| Fluorine | F | Most Non-metallic |
| Chlorine | Cl | |
| Bromine | Br | |
| Iodine | I | |
| Astatine | As | Least non-metallic |
Variation in periods –
On moving from left to right in a period, atomic size gradually decreases. As a result, the force of attraction between the nucleus and valance electrons increases. Hence, the Non-metallic or electronegative character increases as we move from left to right in a period.
OR
The metallic or electropositive character decreases as we move from left to right in a period.
Chemical reactivity –
It depends on position of element in periodic table. / Its outer electron configuration.
In groups – The elements in same group have similar outer electronic configuration. Therefore, elements of same group show same reactivity.
In periods – As we move from left to right, the chemical reactivity first decreases and then increases.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Valance electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| reactivity | Very reactive | Less reactive | less | least | Quite reactive | More | very | non |